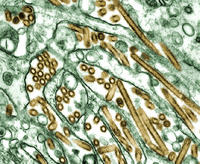
HHS Buys Additional Vaccine As Preparations For Potential Influenza Pandemic Continue
HHS Secretary Mike Leavitt today announced the purchase of additional vaccine that could be used in the event of a potential influenza pandemic.
The department has awarded a $62.5 million contract to Chiron Corporation to manufacture an avian influenza vaccine designed to protect against the H5N1 influenza virus strain, which has caused an epidemic of avian flu in Asia and has recently spread to Europe. The number of individuals who could be protected by the newly contracted vaccine is still to be determined by ongoing clinical studies.
“An influenza vaccine effective against the H5N1 virus is our best hope of protecting the American people from a virus for which they have no immunity,” Secretary Leavitt said. “This contract will increase our stockpile of the vaccine and is a continuation of our aggressive multi-pronged approach to a potentially critical public health challenge.”
This purchase builds on the department’s current plans to buy enough H5N1 influenza vaccine for 20 million people and enough influenza antivirals for another 20 million people. These supplies of vaccine and antiviral treatment will be placed in the nation’s Strategic National Stockpile where they will be available for use should an influenza pandemic occur. Last month, HHS awarded a $100 million contract to sanofi pasteur, the vaccines business of the sanofi-aventis Group, for avian flu vaccine.
Developing an effective avian influenza vaccine is a key element of a comprehensive U.S. approach to prepare for an influenza pandemic that includes improved vaccine production methods and stockpiling of antivirals.
Earlier this year, Secretary Leavitt established an HHS-wide Influenza Task Force to coordinate all HHS activities affecting the public health preparedness for seasonal influenza outbreaks and an influenza pandemic. Long-term objectives include an effective and efficient global surveillance network for outbreaks of influenza-like illness in humans and animals, and interoperable local, state, and federal government response plans for influenza outbreaks within the United States. The task force is also developing strategies to effectively coordinate with response partners, both public and private and insure timely communication with the public.
### FOR IMMEDIATE RELEASE, Thursday, Oct. 27, 2005, Contact: HHS Press Office, (202) 690-6343
RELATED: Sunday, October 16, 2005 FLU VIRUS REPORTED TO RESIST DRUG ENVISIONED FOR PANDEMIC . Sunday, October 09, 2005 Researchers Reconstruct 1918 Pandemic Influenza Virus . Tuesday, August 09, 2005 Bird Flu Cases Increase . Sunday, August 07, 2005 universal flu vaccine . Tuesday, April 05, 2005 Avian Influenza in Asia . Sunday, March 27, 2005 Experimental Avian Flu Vaccine
more at 1918 Pandemic and Influenza Virus or CDC and Pandemic and Avian influenza or Bird Flu and H5N1 or Tamiflu and oseltamivir
Thursday, October 27, 2005
HHS Buys Vaccine Preparations For Potential Influenza Pandemic
Sunday, October 16, 2005
FLU VIRUS REPORTED TO RESIST DRUG ENVISIONED FOR PANDEMIC
FLU VIRUS REPORTED TO RESIST DRUG ENVISIONED FOR PANDEMIC, RELATED: Researchers Reconstruct 1918 Pandemic Influenza Virus
Scientists from the University of Wisconsin-Madison, working with colleagues in Vietnam and Japan, report in a brief communication in next week's edition (Oct. 20, 2005) of the journal Nature that a young girl, provided with a prophylactic dose of the drug after experiencing mild influenza symptoms, developed a strain of the virus that was highly resistant to the drug.The finding suggests that health officials - now stockpiling millions of doses of the drug to forestall a global outbreak of influenza and buy time to develop and mass produce a vaccine - should also consider other options, according to Yoshihiro Kawaoka, an international authority on influenza and the senior author of the Nature paper.
Recent reports indicate the federal government may spend billions of dollars to stockpile as much as 81 million courses of Tamiflu to forestall a possible influenza pandemic. The government has already stockpiled an estimated 12 to 13 million courses.
"This is the first line of defense," says Kawaoka, a professor in the UW-Madison School of Veterinary Medicine who holds a joint appointment at the University of Tokyo. "It is the drug many countries are stockpiling, and the plan is to rely heavily on it."
The drug would be used to slow the spread of influenza until a vaccine is developed, which may take up to six months.
Tamiflu is delivered orally and works to impede the spread of the virus by binding to and inhibiting one of the surface enzymes the virus uses to exit infected cells of a host. Once inside a host cell, the virus commandeers the cell's reproductive machinery to make new infectious particles that go on to take over other cells. When the drug is at work, Kawaoka explains, "the virus is still able to replicate inside a cell, but is unable to get out and infect other cells."
Oseltamivir, which Kawaoka describes as an "amazing drug," is one of three compounds proven to be effective against influenza. One class, derivatives of the compound adamantine, would be less effective, as some flu viruses have already evolved resistance to it. The other drug, zanamivir, which was developed prior to oseltamivir, is effective, but is formulated as a powder and requires that a clinician provide instructions for use. Thus, it is more cumbersome to administer than the orally delivered Tamiflu.
These flu-fighting drugs, says Kawaoka, are by no means a replacement or alternative to a vaccine. Effective vaccines can confer immunity, preventing the virus from gaining a toehold in the body. But it is unlikely sufficient quantities of a vaccine can be produced and stockpiled prior to the emergence of a new virus in human populations.
If avian influenza does emerge and becomes infectious from human to human - and nearly all experts agree that will happen at some point in the future - an outbreak similar to the 1918 influenza pandemic could occur. That pandemic killed as many as 50 million people, more than died on all the battlefields of World War I. Scientists and vaccine manufacturers would be in a race against time to produce enough doses to forestall disaster. Drugs like Tamiflu, used in combination with quarantine, would be intended to slow the spread of the disease until a vaccine is produced.
Kawaoka says there may not be enough Tamiflu to go around even though countries are stockpiling it. The Wisconsin scientist says that will create a risk of patients sharing the drug and using smaller doses, which could accelerate the emergence of virus resistant to the drug and hamper efforts to contain the spread of the disease.
He says health officials should consider stockpiling zanamivir and recommending that only the therapeutic dosages of Tamiflu be administered to patients.
"We've been watching for this change (in the virus)," Kawaoka says. "This is the first, but we will see others. There's no question about it." ###
- Terry Devitt, (608) 262-8282, trdevitt@wisc.edu FOR IMMEDIATE RELEASE 10/14/05
CONTACT: Yoshihiro Kawaoka, (608) 265-4925, kawaokay@svm.vetmed.wisc.edu
more at 1918 Pandemic and Influenza Virus or CDC and Pandemic and Avian influenza or Bird Flu and H5N1 or Tamiflu and oseltamivir
Sunday, October 09, 2005
Researchers Reconstruct 1918 Pandemic Influenza Virus
Researchers Reconstruct 1918 Pandemic Influenza Virus; Effort Designed to Advance Preparedness
“This groundbreaking research helps unlock the mystery of the 1918 flu pandemic and is critically important in our efforts to prepare for pandemic influenza,” said CDC Director Dr. Julie Gerberding. “We need to know much more about pandemic influenza viruses. Research such as this helps us understand what makes some influenza viruses more harmful than others. It also provides us information that may help us identify, early on, influenza viruses that could cause a pandemic.”The work, done in collaboration with Mount Sinai School of Medicine, the Armed Forces Institute of Pathology and Southeast Poultry Research Laboratory, determined the set of genes in the 1918 virus that made it so harmful. Prior to this study, which is published in the Oct. 7 issue of Science, flu experts had little knowledge of what made the 1918 pandemic so much more deadly than the 1957 and 1968 pandemics. This week’s issue of Nature also includes a related article entitled “Characterization of the 1918 influenza virus polymerase genes” which describes the final three gene sequences of the 1918 influenza virus. The work reported in the Nature article was done by scientists at the Armed Forces Institute of Pathology.
The 1918 pandemic killed an estimated 20-50 million people worldwide, including 675,000 in the United States. The pandemic’s most striking feature was its unusually high death rate among otherwise healthy people aged 15-34. During normal seasonal flu outbreaks, severe complications and death are most common among the elderly and young children.
Influenza pandemics occur when a new strain emerges to which people have little or no immunity. Most experts believe another pandemic will occur, but it is impossible to predict which strain will emerge as the next pandemic strain, when it will occur or how severe it will be.
“By identifying the characteristics that made the 1918 influenza virus so harmful, we have information that will help us develop new vaccines and treatments,” said Dr. Terrence Tumpey, the CDC senior microbiologist who recreated the virus. “Influenza viruses are constantly evolving, and that means our science needs to evolve if we want to protect as many people as possible from pandemic influenza.”
In reconstructing the 1918 influenza virus, researchers learned which genes were responsible for making the virus so harmful. This is an important advance for preparedness efforts because knowing which genes are responsible for causing severe illness helps scientists develop new drugs and vaccines (e.g., they can focus their research on those genes).
CDC employed stringent biosafety and biosecurity precautions during research on the 1918 influenza virus. The work was done in a high containment Biosafety Level 3 lab with enhancements that include special provisions to protect both laboratory workers and the public from exposure to the virus. Currently available antiviral drugs have been shown to be effective against influenza viruses similar to the 1918 influenza virus.
All laboratory work was conducted at CDC. The work was supported in part with funding from the U.S. Department of Agriculture and the National Institutes of Health.
To evaluate the benefits of publishing the information contained in these manuscripts and any potential threat from its possible deliberate misuse, both manuscripts were reviewed by the National Science Advisory Board on Biosecurity (NSABB). The NSABB advises the federal government on strategies for the conduct and communication of research that might yield information or technologies that could be misused to threaten public health or national security. The Board was unanimous in its determination that it was critically important to make these findings available to the scientific community at large to not only validate their significance, but also permit further research on the development of diagnostic tests, treatments, and preventative measures.
Source: CDCFor Immediate Release, October 5, 2005 Contact: CDC Media Relations, 404-639-3286
more at 1918 Pandemic and Influenza Virus or CDC and Pandemic and Avian influenza or Bird Flu and H5N1
Tuesday, August 09, 2005
Bird Flu Cases Increase
Vietnam has detected a total of 90 human cases of H5N1 since the disease first began to appear in the region in late 2003. Of those, 40 have died.
WHO’s official accounting of human cases issued August 5 tallies 112 in four nations – Vietnam, Thailand, Cambodia and Indonesia. After Vietnam, Thailand has confirmed the most avian influenza cases – 17 – while Indonesia is the most recent government to report a human death. A man who died July 12 had two young daughters who also became ill and subsequently died. Tests are still ongoing to determine whether H5N1 was the cause of the girls’ deaths.
As the human toll of the disease increases, so does the spread of the virus among bird populations. Russian animal health officials have reported to the World Organisation for Animal Health the appearance of H5N1 in three villages in Novosibirsk province.
This Russian region borders on Kazakhstan, where a strain of bird flu is also reported, according to news reports, but not yet confirmed as the highly dangerous H5N1 strain.
This strain infected humans for the first time in only 1997, health officials say, so immunity to it is virtually nonexistent in people. The pattern of human infection so far proves that the virus is not easily transmitted between humans. Most cases have been traced to close contact with infected birds.
Health authorities fear though that H5N1 will mutate to become more transmissible between humans. If that happens, in a world of rapid transit and globalized travel, experts say a flu pandemic could sweep from nation to nation with the potential death toll in the tens of millions, and economic and trade disruption of immense proportions.
Pandemic Research
A timely response with a targeted distribution of antiviral drugs could contain an epidemic and prevent a global spread, according to research published by international research teams. Using computer models, the research shows that pandemic could be prevented with a combination of carefully implemented public health measures introduced soon after the first cases appear.
Scientists in the United States, Hong Kong, Thailand and France produced the work as participants in a research network funded by the National Institute of General Medical Sciences (NIGMS), a component of the National Institutes of Health.
Two different computer models tested different outbreak scenarios, according to an August 3 NIGMS press release. One focused on 85 million people in Thailand and bordering regions of neighboring countries. Published in the magazine Nature, this study found that 3 million courses of antiviral drugs targeted for treatment of infected individuals and all their contacts – family, friends, schoolmates, coworkers, and shopkeepers – could have more than a 90 percent chance of stopping the virus.
A second computer model developed a scenario involving 500,000 people in rural Southeast Asia. Described in the magazine Science, this model applied similar treatment and response strategies to those of the first study, but also called for the pre-pandemic inoculation of the population with a flu vaccine, even though the vaccine would be considered low-efficacy. That is, it would be a vaccine of limited value because it would not have been specifically developed to target a rapidly emerging, previously unknown viral strain.
An inoculation campaign would help bolster the effectiveness of the other containment strategies such as quarantine and antiviral treatment according to the study. Under that scenario, the spread of the pandemic might be contained to less than one case per 1,000 people.
Further information on both studies is available at the MIDAS (Models of Infectious Disease Agent Study) Web site.
more at avian influenza or bird flu and H5N1 or pandemic and World Health Organization or National Institutes of Health
Sunday, August 07, 2005
universal flu vaccine
VIB signs cooperation agreement for the development of a new, universal flu vaccine
Ghent, Belgium – This week the Flanders Interuniversity Institute for Biotechnology (VIB) has entered into an agreement with the British company Acambis for the development of a new flu vaccine. Together VIB and Acambis want to develop a universal flu vaccine that offers protection from all flu variants. Furthermore this vaccine will not need to be renewed annually. VIB researchers linked to the Ghent University - led by Walter Fiers, professor Molecular Biology and former director of one of the Ghent VIB departments - carried out the research on which this new flu vaccine is based.
Flu, not an innocent illness...
Flu is an acute infection of the bronchial tubes and is caused by the flu virus. Flu is extremely contagious and makes you feel really ill. In addition, flu can have extremely serious consequences: on average 1,500 people die of flu in Belgium annually. There were even as many as 4,500 flu victims during the winter period of 1989 and 1990. According to the World Health Organization, on average 10 to 20% of the world's population is infected by flu every year. This leads to 3 to 5 million hospitalizations and 250 000 to 500 000 deaths a year.
What makes the influenza virus so special?
The outer coating of the influenza virus changes gradually, making the virus invisible to the antibodies that were built up during an earlier infection or vaccination; after all, these antibodies are aimed at the changeable proteins of the outer coating. Since no one as yet has antibodies, the slightly altered virus can easily set off a new epidemic. Thus far no vaccine has been developed against influenza that lasts for a lifetime, in contrast to diseases as polio, hepatitis B or measles.
Flu, prevention is better...
Vaccination offers the most efficient protection to flu. The present flu vaccines are a mixture of three vaccines, each offering protection to a certain virus strain. Vaccination reduces death and the amount of hospitalizations as a result of flu considerably. The government strongly advises flu vaccination for those over 60 years old, diabetes patients, people with diminished resistance or chronic kidney, heart and lung disorders. However, everyone can consider vaccination: it will give you 80% less chance of getting the flu next winter.
No more annual renewal thanks to new research?
VIB and the British company Acambis are collaborating on the development of a universal flu vaccine that offers protection to all flu variants. Moreover, the vaccine would not require annual adaptations to the virus changes. This is because the candidate vaccine uses the M2e-domain. This domain is strongly conserved, and nearly identical in all human virus strains, also those that caused the pandemics in the previous century. As a consequence, people can be vaccinated long before a new pandemic breaks out.
This innovative vaccine is currently in a preclinical phase. It is not longer produced by the growth of viruses, but the active component is produced in bacteria. Therefore the production process will be more effective, safer and cheaper.
Walter Fiers, co-inventor (UGent and VIB):
'The structure of M2e is almost identical in all known flu viruses that can be transmitted between people. We indicated that the M2e vaccine offers full protection to flu without side effects in mice. This way vaccination against all human flu viruses is possible, not only against viruses returning yearly, but even against future epidemics. Through our cooperation with Acambis we can continue the development of this promising vaccine.'
Cooperation between VIB and Acambis
Professor Walter Fiers and his team carried out the research on which the collaboration between VIB and Acambis is based. Acambis recently acquired an influenza A vaccine candidate from Apovia, a US company. Apovia had originally obtained the necessary technology for the development of the vaccine from VIB. Through the research collaboration with VIB, together with internal research, Acambis enters the flu vaccine market, one of the most significant vaccine markets.
Gordon Cameron, CEO (Acambis):
'This programme gives Acambis the opportunity to enter one of the most significant vaccine markets - influenza. The recent influenza vaccine shortages have highlighted the inadequacies of current influenza vaccines and their manufacturing methods. Through the collaboration with VIB we get the chance to develop the ultimate vaccine that will offer protection against all flu virus variants and reduce the annual redesign of the vaccine.' ###
Background information
How do influenza vaccines work?
Each year the World Health Organization determines which three influenza viruses are the most likely to cause an influenza epidemic during the next winter period. These candidates are used for the production of a vaccine. These days influenza vaccines are prepared by growing viruses on chicken embryos or in cultures of animal cells. Then proteins originating from the outer coating of the virus are purified from the harvested viruses and used as vaccine. After inoculation, the human immune system creates antibodies. After a few weeks, sufficient antibodies have been produced and will neutralize the viruses in case of an infection. Since new species of viruses that have slightly altered proteins on their outer coatings appear nearly every year, injection must be repeated every year.
Pandemics, a tremendous threat
A new human influenza virus in which the outer coating is drastically altered appears a few times every century. This new virus spreads lightning-fast around the entire world, since no one has yet been able to build up antibodies against it. Such a worldwide epidemic or pandemic can contaminate up to 50% or more of the world population. In the last century there was the Asian flu, the Hong Kong flu and above all the Spanish flu. During the period between 1917 and 1920 the latter was responsible for over 50 million victims.
Pandemic viruses occur when the genetic material originating from a fowl plague virus is mixed with that of a human influenza virus. This is why the World Health Organization has followed the recent epidemics caused by the fowl plague virus of the type H5N1 in the Far East with an extremely watchful eye.
The virus species that will cause the next pandemic is not known, so no vaccine can be produced against it in advance. Only after a pandemic has burst into action, the responsible virus can be identified and a suitable vaccine can be developed. It will take some 6 to 9 months before the vaccine is ready for use. 'Optimistic' predictions speak of two million deaths worldwide and 20,000 deaths in Belgium.
Contact: Karine Clauwaert karine.clauwaert@vib.be 32-9-244-6611 VIB, Flanders Interuniversity Institute of Biotechnology
more at influenza or Flu and Molecular Biology or immunology and pandemic or Medicine
Tuesday, April 05, 2005
Avian Influenza in Asia
Avian Influenza in Asia
The U.S. Government is concerned about the ongoing avian influenza, or bird flu, outbreak in Asia and its potential for becoming a human flu pandemic. From January 2004 until now, outbreaks of bird flu–formally known as Avian Influenza H5N1–have been confirmed among poultry in Vietnam, Cambodia, China, Indonesia, Japan, Laos, Malaysia, South Korea, and Thailand. North Korea recently reported incidents of avian influenza, but the influenza strain remains uncertain.
Avian influenza has killed nearly 50 people in Southeast Asia and resulted in the deaths of millions of poultry. Current influenza treatments for human cases are unproven and medical professionals warn of a global pandemic if the virus develops the capacity to be transmitted easily from person to person. However, the vast majority of the known human cases have resulted from direct contact with poultry and there is only limited evidence to suggest human-to-human transmission.
The United States is collaborating closely with the World Health Organization, the World Organization for Animal Health, and the Food and Agricultural Organization of the United Nations to address the situation. These organizations, working with their members, are offering technical assistance to affected countries throughout the region. At the request of the World Health Organization, the United States provided three test kits to the organization for use in North Korea to determine what influenza viruses are causing outbreaks in poultry and to test if humans have been infected. The U.S. Government approved the release of the kits on a humanitarian basis to help the people of North Korea.
The United States is also offering bilateral technical and epidemiological help to select countries through the Departments of Agriculture and Health and Human Services and the U.S. Agency for International Development. Over the past year, the Department of Health and Human Services provided over $5.5 million in technical help and grants to the region and the World Health Organization for influenza pandemic preparedness, including emergency support in the form of experts and laboratory reagents from its Centers for Disease Control and Prevention. The Department of Agriculture, with the Asia-Pacific Economic Cooperation forum, is organizing a symposium on avian influenza response, preparedness, and human health emergency in San Francisco in July. The U.S. Agency for International Development has sent stocks of personal protective equipment to the region to be used if an outbreak begins to spread rapidly.
To help protect Americans at home and abroad, the State Department has issued a Fact Sheet informing the public about avian influenza. For more information go to state.gov/avianflu 2005/377 Released on April 5, 2005 Press Statement Richard Boucher, Spokesman Washington, DC April 5, 2005
Sunday, March 27, 2005
Experimental Avian Flu Vaccine
NIAID Initiates Trial of Experimental Avian Flu Vaccine, Fast-track recruitment has begun for a trial to investigate the safety of a vaccine against H5N1 avian influenza, the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), announced today.
Sites in Rochester, NY, Baltimore and Los Angeles will enroll a total of 450 healthy adults. The clinical sites are part of the NIAID-sponsored Vaccine and Treatment Evaluation Units (VTEU).
“While there have been relatively few cases worldwide of H5N1 avian influenza infection in humans, the public health community is concerned that the virus will develop the capability of efficiently spreading from human to human and thus create a risk for a worldwide pandemic,” says NIAID Director Anthony S. Fauci, M.D.
“NIAID has supported research on H5N1, the strain responsible for this deadly form of avian influenza, since 1997 when the first cases in humans were reported. The initiation of this vaccine trial marks a key advance in our efforts to prepare to respond to an avian flu pandemic,” adds Dr. Fauci.
Sanofi pasteur, Swiftwater, PA, manufactured the trial vaccine, which is an inactivated vaccine made from an H5N1 virus isolated in Southeast Asia in 2004. Sanofi pasteur, formerly Aventis Pasteur, was awarded a contract by NIAID to manufacture the H5N1 vaccine in May 2004.
This Phase I trial will test the vaccine’s safety and ability to generate an immune response in 450 healthy adults aged 18 to 64. If the vaccine is shown to be safe in adults, there are plans to test it in other populations, such as the elderly and children.
H5N1 avian influenza leads to severe disease in both birds and humans. Between January 2004 and March 11, 2005, there were 69 confirmed cases of and 46 deaths from H5N1 infection in humans reported to the World Health Organization. To date, there has been a small number of cases where human-to-human transmission of the virus may have occurred. However, public health experts fear that the virus may evolve into one that is more easily transmitted between people. If this were to happen, a worldwide pandemic could follow.
Influenza pandemics are global outbreaks that emerge infrequently and unpredictably and involve strains of virus to which humans have little or no immunity. H5N1 is one such flu virus strain. The last influenza pandemic swept the globe in 1968; many public health officials believe the world is overdue for another one.
The VTEUs now enrolling adult volunteers are
- University of California at Los Angeles (Joel Ward, M.D., Principal Investigator)
- University of Maryland School of Medicine, Baltimore, MD (James Campbell, M.D., P.I.)
- University of Rochester School of Medicine and Dentistry, Rochester, NY (John Treanor, M.D., P.I.)
NIAID is a component of the National Institutes of Health, an agency of the U.S. Department of Health and Human Services. NIAID supports basic and applied research to prevent, diagnose and treat infectious diseases such as HIV/AIDS and other sexually transmitted infections, influenza, tuberculosis, malaria and illness from potential agents of bioterrorism. NIAID also supports research on transplantation and immune-related illnesses, including autoimmune disorders, asthma and allergies. SOURCE: National Institute of Allergy and Infectious Diseases (NIAID)
FOR IMMEDIATE RELEASE Wednesday, March 23, 2005 CONTACT: Anne A. Oplinger 301- 402-1663