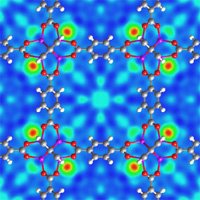
Using beams of neutrons as probes, NIST scientists determined where hydrogen latches onto the lattice-like arrangement of zinc and oxygen clusters in a custom-made material known as a metal-organic framework, or MOF. Called MOF5, the particular nanoscale material studied by Taner Yildirim and Michael Hartman has four types of docking sites, including a "surprising" three-dimensional network of "nano-cages" that appears to form after other sites load up with hydrogen.
This finding, reported in Physical Review Letters,* suggests that MOF materials might be engineered to optimize both the storage of hydrogen and its release under normal vehicle operating conditions. It also suggests that MOFs might be used as templates for interlinking hydrogen nano-cages, creating materials with unusual properties due to a phenomenon known as quantum confinement. In a sense, this discovery is a bonus.
Yildirim and Hartman found that the two most stable sites in the scaffolding already offer considerable room for storing hydrogen, accounting for the interest MOFs already have attracted. Earlier studies reported that, at about –200 degrees Celsius, MOF5 could hold less than 2 percent of its weight in hydrogen.
The NIST research indicates ample room for improvement. At very low temperatures, hydrogen uptake approached 10 percent of the material's weight. (The FreedomCar and Fuel Partnership involving the Department of Energy and the nation's "Big 3" automakers has set a level of about 6 percent as a minimum capacity for economically viable hydrogen storage.) The bulk of the hydrogen was held in nanometer-scale cavities inside the box-like arrangements of zinc and oxygen clusters.
"Neutron diffraction measurements clearly show that the molecules are packed in a fashion similar to the way apples or oranges fill a bowl," Yildirim explains. The unexpected nano-cages introduce the potential for spillover capacity, so to speak.
Hydrogen storage levels of 10 percent are encouraging, but these results were achieved at impractically low temperatures. Yildirim and Hartman say they hope better understanding of how hydrogen molecules tether to MOFs will ultimately lead to improved materials suitable for practical applications. ###
The research was carried out at the NIST Center for Neutron Research and partially supported by the U.S. Department of Energy. More information can be obtained at ncnr.nist.gov/staff/taner/h2.
*T. Yildirim and M.R. Hartman, Direct observation of hydrogen adsorption sites and nano-cage formation in metal-organic frameworks (MOF). Phys. Rev. Lett., 95, 215504 (2005).
Contact: Mark Bello mark.bello@nist.gov 301-975-3776 National Institute of Standards and Technology (NIST)
more at Nano or Chemistry and mechanical engineering or computer science and computer engineering or Nanotechnology and Nanotech or Science and macrophages or hydrogen and NIST or nanoscale
Related: Keyword Nanotech Sunday, November 13, 2005 Testing toxicity of nanomaterials, Sunday, October 23, 2005 single-molecule car, 'Nanocar', Sunday, August 28, 2005 Writing at the nanoscale, Thursday, May 26, 2005 discontinuous palladium, siloxane self-assembled monolayer, Sunday, May 08, 2005 Center for Nanoscale Materials, Monday, April 25, 2005 Nanomagnets, Nanocomposite, Monday, March 21, 2005 porphyrin tubes may lead to new nanodevices, inexpensive hydrogen fuel